Breastfeeding is a standardized feeding method for infants and a public health issue as it can improve infant survival rates and maternal health. However, 5-15% of women worldwide suffer from breast developmental abnormalities and lactation dysfunction. The Notch signaling pathway has been widely reported to be involved in the maintenance of breast stem cells, determination of cell fate, and differentiation during breast development and maturation. The activation of Notch1 in breast stem cells is necessary for promoting the differentiation of breast cells and facilitating the gradual transformation of monoenergetic estrogen receptor negative breast cells.
Kindlin-2 is an important integrin binding protein encoded by the FERMT2 gene, which is highly expressed in organs derived from the mesoderm, including smooth muscles, heart, blood vessels, and bones. Myoepithelial cells play an important role in breast development, but the molecular mechanisms by which myoepithelial cells control acinar cell differentiation during pregnancy are still unclear.
In October 2023, the research team led by Zhang Hongquan and Zhan Jun from Peking University School of Basic Medicine and Peking University International Cancer Institute published a research paper titled "Kindlin-2 in myoepithelial controls luminal progenitor commitment to alveoli in mouse mammary gland" in the journal Cell Death&Disease.
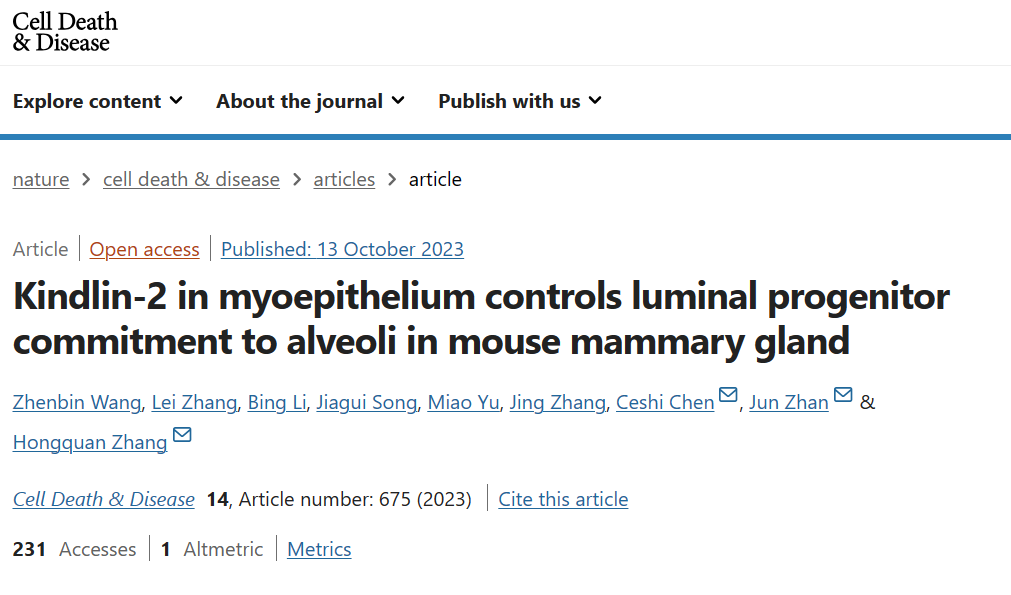
This study found that the absence of Kindlin-2 in myoepithelial cells can impair breast morphogenesis, acinar cell formation, and lactation. Using five genetically modified mouse models combined with single-cell RNA sequencing, we found a Kindlin-2-Stat3-Dll1 signaling cascade in myoepithelial cells that can inactivate the Notch signaling pathway in luminal cells, thereby promoting the differentiation of luminal progenitor cells into acinar cells. Single cell spectrum analysis showed that Kindlin-2 deficiency significantly reduced the proportion of mature alveolar cells. In terms of molecular mechanisms, the absence of Kindlin-2 in myoepithelial cells promotes the activation of Stat3 and upregulation of Dll1, which activate the Notch signaling pathway in luminal cells and inhibit the differentiation and maturation of luminal progenitor cells during pregnancy.
Inhibition of Notch1 with hesperidin restored differentiation ability of luminal progenitor cells in pregnant mice with Kindlin-2 deficiency in myoepithelial cells. In summary, the article demonstrates that Kindlin-2 is an essential factor for myoepithelial cells to control the transformation of luminal progenitor cells into acinar cells during pregnancy.
rimental part
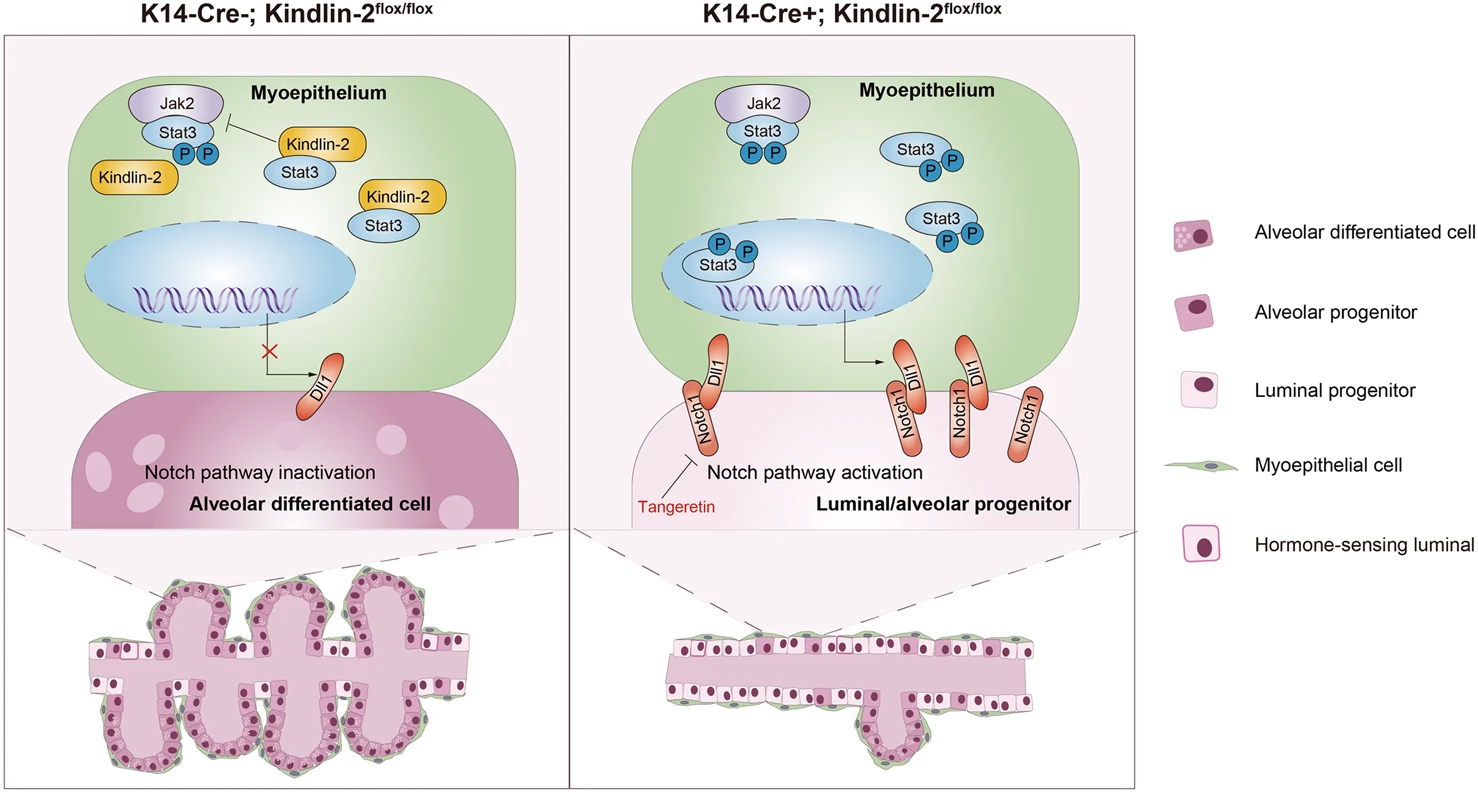
Due to the fact that myoepithelial cells are a special type of muscle like differentiated epithelial cell, they are often thinly attached to luminal epithelial cells, resulting in a low proportion of their existence. In traditional analysis processes, there is interference with luminal epithelium, making it difficult to accurately quantify analysis. Therefore, researchers have concluded through extensive preliminary research that the resolution of traditional sequencing methods cannot meet the requirements, and have turned to single-cell multi omics quantitative analysis techniques to achieve in-depth analysis of their expression and distribution without damaging the original tissue structure and morphology. In order to ensure the accuracy and reliability of single-cell multi omics quantitative analysis data, all immunofluorescence images and quantitative analysis results in this article were analyzed and validated using Tissue Cytometry technology from TissueGenomics.
Panel 1: CK5,CK8,Kindlin-2
In terms of experimental details, the researchers first focused on the physiological distribution of Kindlin-2 in the mammary gland and found that Kindlin-2 was highly expressed in CK5 labeled myoepithelial cells. It was also confirmed that its expression was significantly reduced in gene knockout mice, establishing a research foundation for subsequent experiments.(Panel1)
Panel 2: CK5,DII1,CK8,Notch1
Next, in order to detect Dll1 in the myoepithelium and Notch1 in the luminal epithelium, it was further confirmed in situ that the absence of Kindlin-2 in the myoepithelium activates the Notch signaling pathway between the myoepithelium and luminal epithelium, which is consistent with the results of single-cell pseudo temporal analysis, The researchers used multiple immunofluorescence quantitative analysis techniques to determine whether the signaling pathway between myoepithelium and luminal epithelium is achieved through contact at the single-cell recognition level through location analysis.(Panel2)
Panel 3: CK5,STAT3,STAT3 pY705,DII1
Finally, to confirm the in situ regulatory interaction between Kindlin-2 and Stat3 in the breast microenvironment, Researchers used fluorescent multi-color immunolabeling technology to detect the phosphorylation levels of Dll1, Stat3 Y705, and S727 sites in the mammary gland of E18.5 myoepithelial deficient Kindlin-2 mice (K14 Cre+; Kindlin-2flox/flox), and confirmed the existence of Kindlin-2-Stat3-Dll1 signaling cascade in tissue in situ.(Panel3)
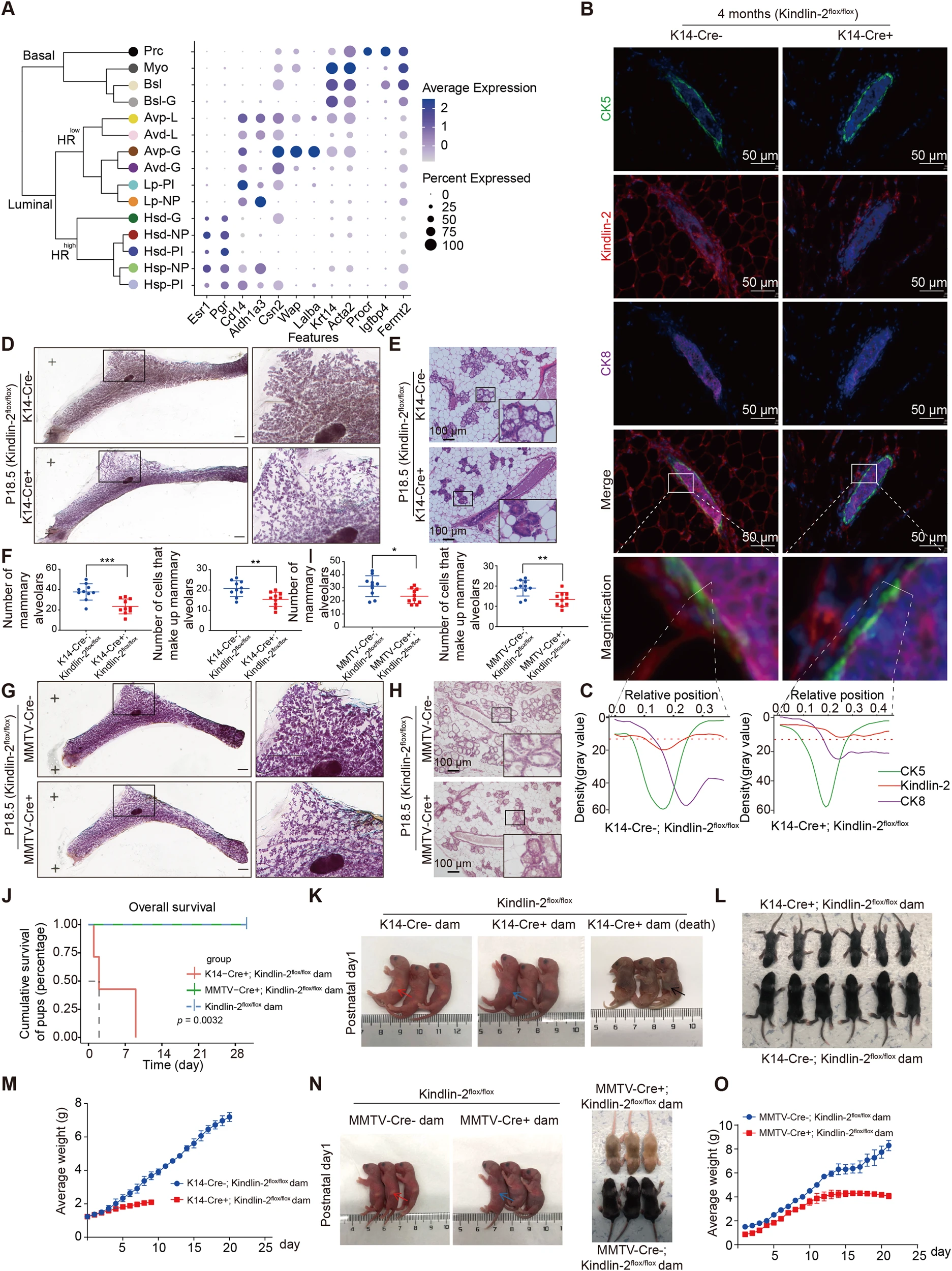
Figure 1 Knocking out Kindlin-2 on mammalian skin inhibits lactation in female mice
B:K14-Cre + ; Kindlin-2flox/flox or K14-Cre-; Multicolor immunofluorescence images of CK5 (green), Kindlin-2 (red), CK8 (purple), and DAPI (blue) in the mammary glands of 4-month-old mice in the Kindlin-2flox/flox control group.
Figure 2 Kindlin-2 specific knockout of myoepithelial cells activates the Notch signaling pathway in luminal epithelium by upregulating Dll1 in P18.5 mammary gland and Notch1
D:CK5 (green), Dll1 (red), CK8 (purple), Notch1 (yellow), and DAPI (blue) of P18.5 mammary gland in K14 Cre+; Kindlin-2flox/flox or K14 Cre -; Multicolor immunofluorescence images of Kindlin-2 flox/flox littermates control mice.
G:Statistical analysis shows the percentage of Dll1+cells in CK5+cells and Notch1+cells in CK8+cells at P18.5 in K14 Cre+cells; Comparison between Kindlin-2flox/flox group and K14 Cre - group.
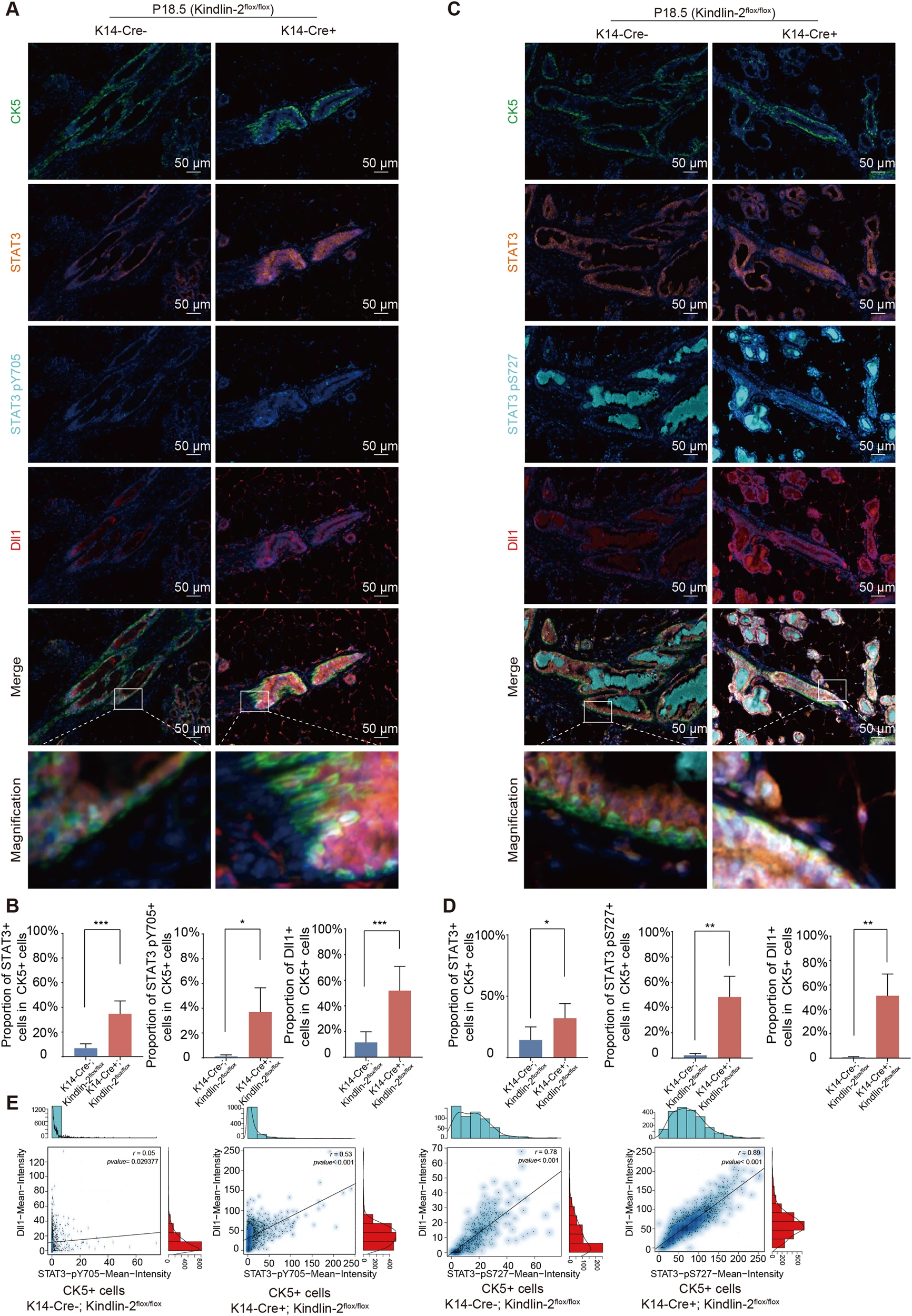
Figure 3 The absence of Kindlin-2 activates STAT3 in the myoepithelium and upregulates Dll1.
A:K14-Cre+;Kindlin-2 Flox or K14-Cre-;Kindlin-2 Flox/FloxMulticolor immunofluorescence images of CK5 (green), STAT3 (orange), STAT3 pY705 (cyan), DL11 (red), and DAPI (blue) in female P18.5 mammary gland
B:Statistical analysis shows the percentage of STAT3+cells in CK5+cells, STAT3 pY705+cells in CK5+cells, and Dll1+cells in CK5+units at P18.5 in K14 Cre+cells; Comparison between Kindlin-2flox/flox group and K14 Cre - group; Kindlin-2 flox/flox group.
***It is worth noting that in the discussion part of the article, the researchers proposed that further research is needed in the future to determine whether the regulatory interaction between the Kindlin-2 and Jak2-Stat3-Dll1 axes, as well as the complex regulatory process between the Kindlin-2, Jak Stat signaling pathway (related to inflammation) and Notch signaling pathway (related to cell dryness and fate differentiation), is related to breast related diseases such as mastitis and breast cancer. These deeper exploration contents can still be achieved through the cell tissue spatial interaction network research module in Tissue Cytometry technology.