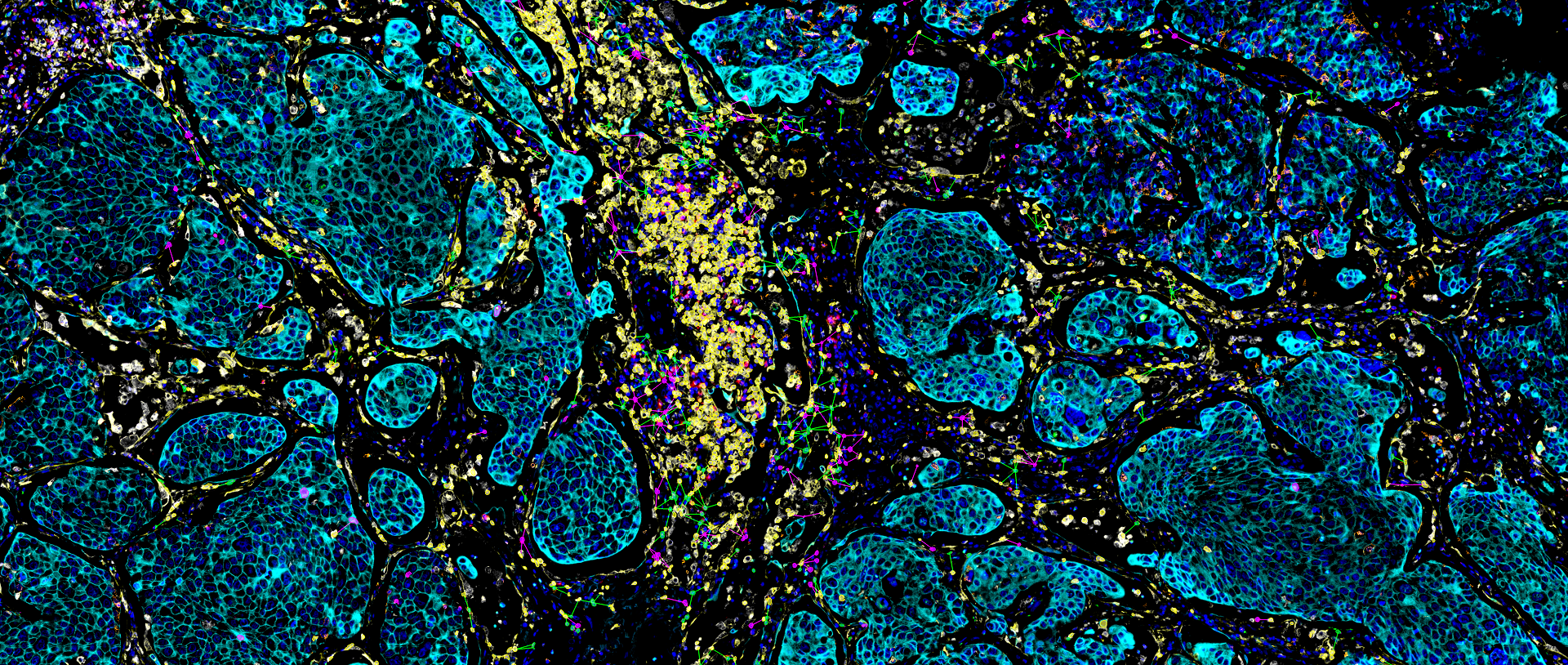
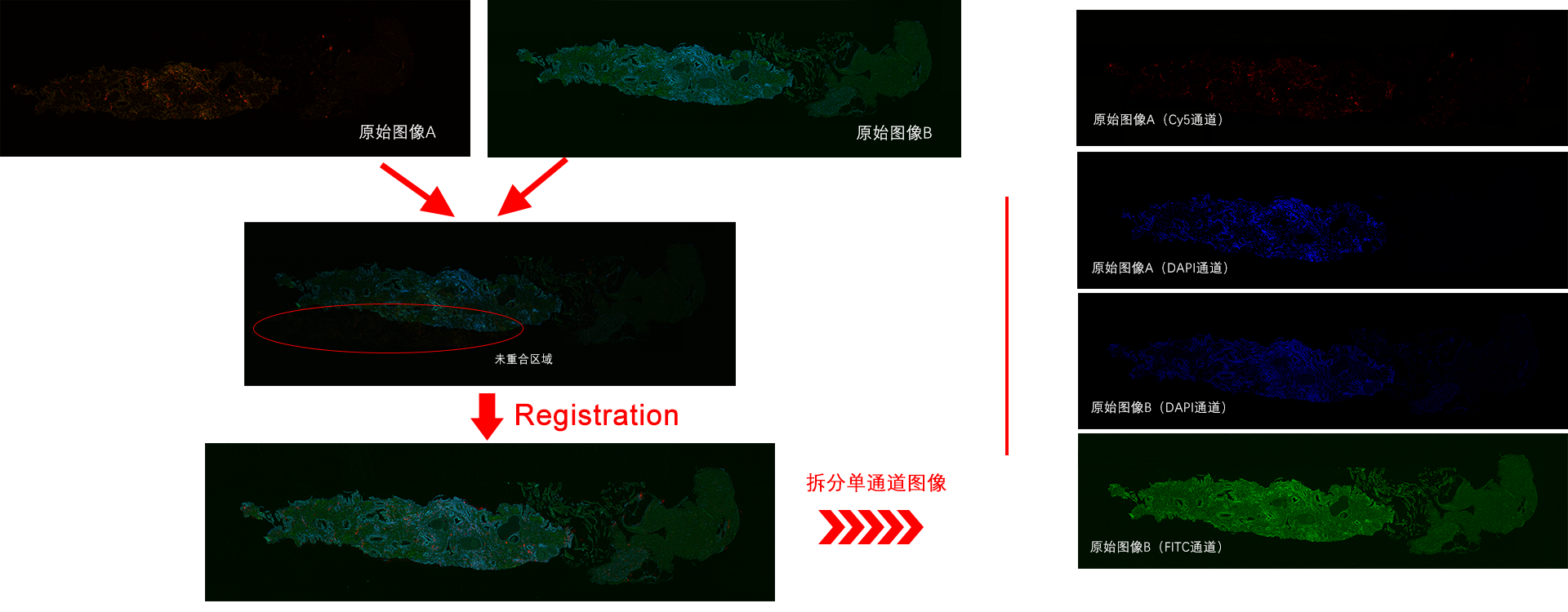
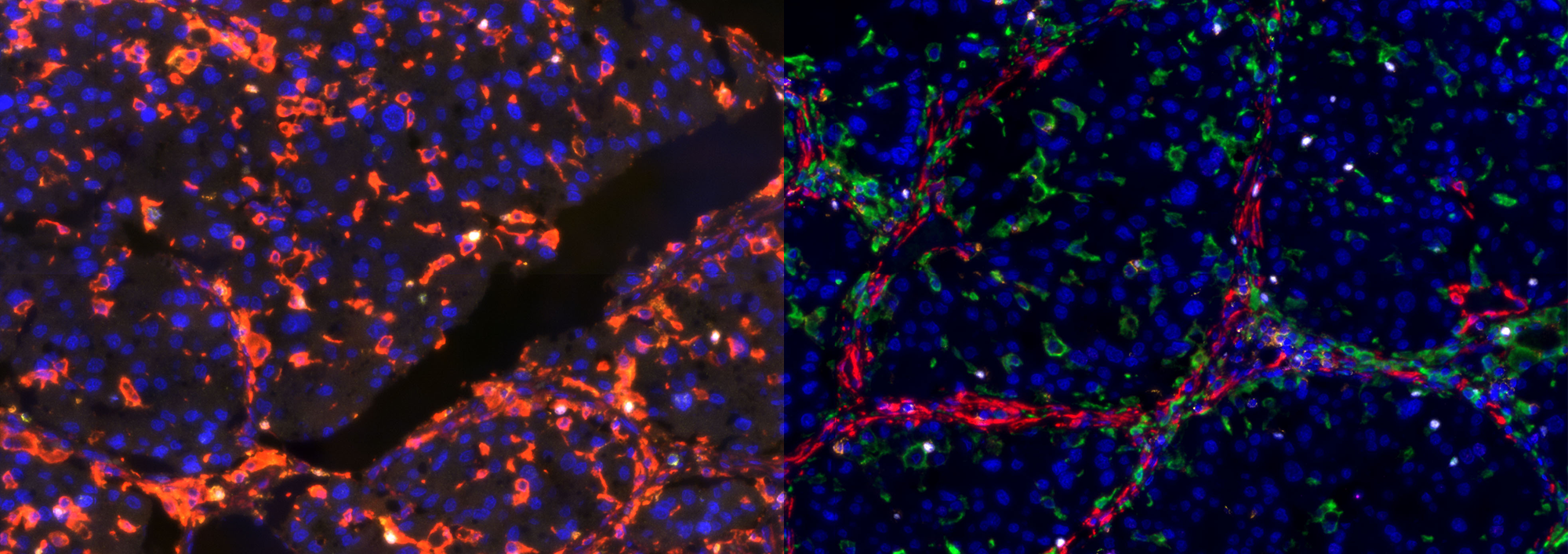
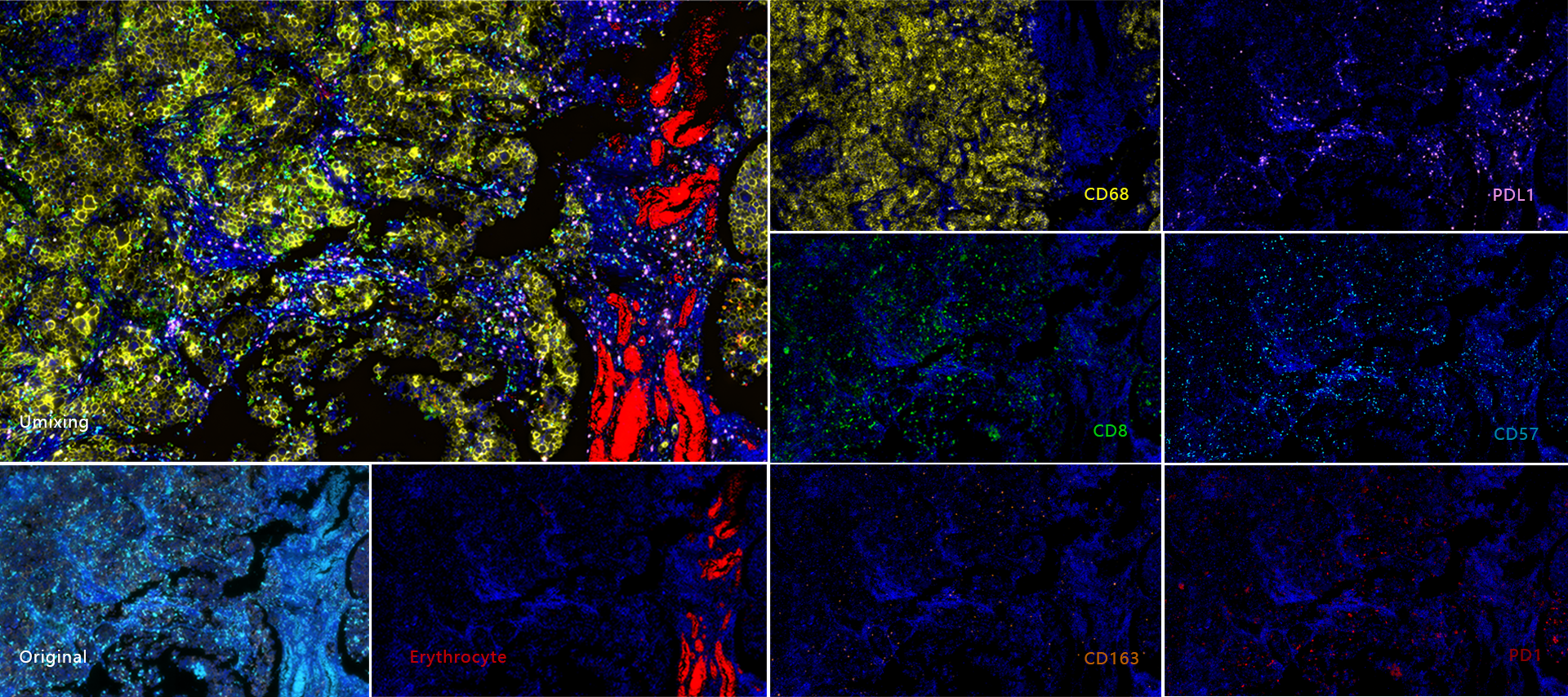
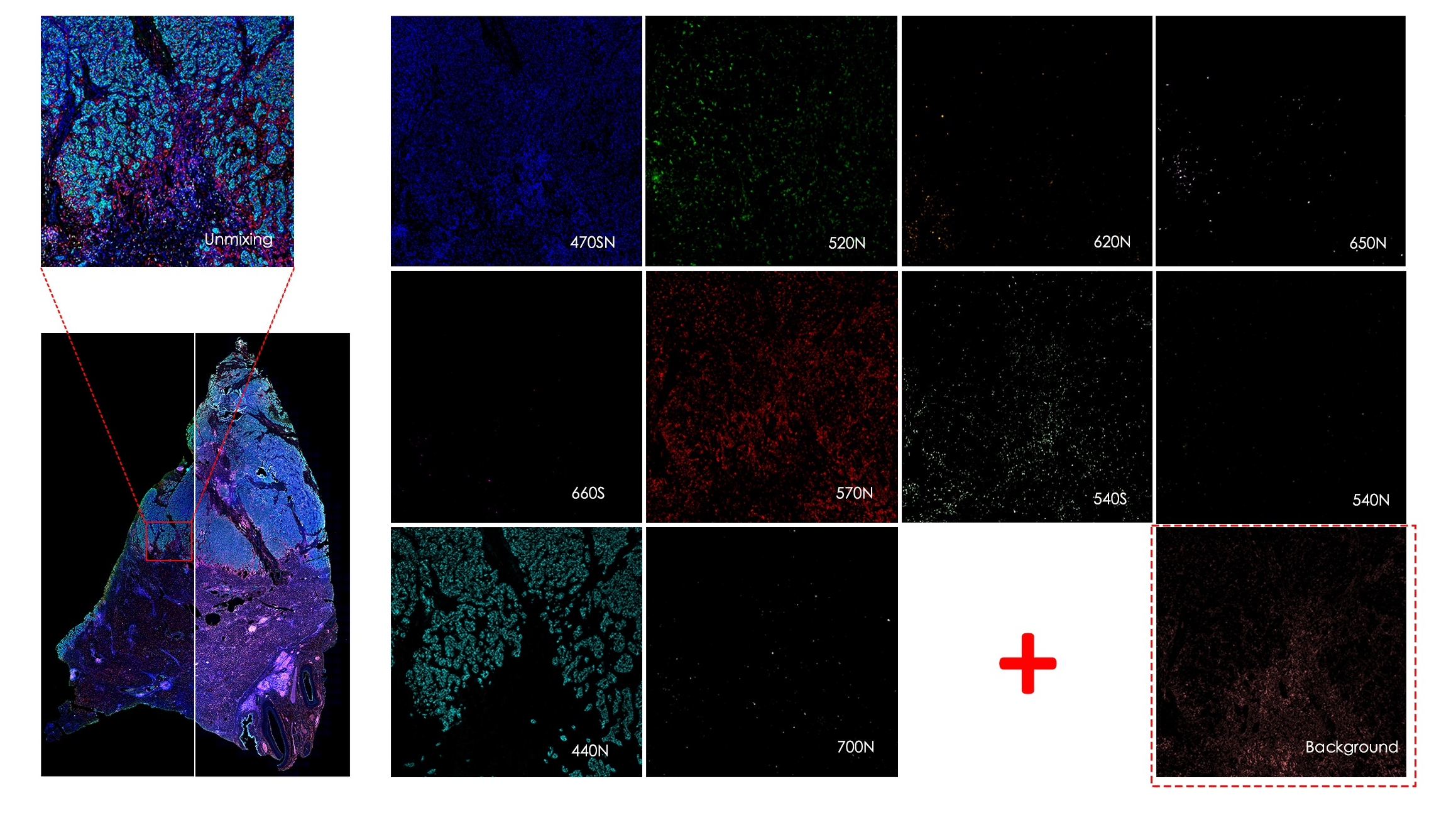
TissueFAXS Spectra enables multi-color panoramic continuous spectral imaging, multiplex fluorescence staining spectral unmixing, and the removal of autofluorescence from blood cells or samples. It overcomes the limitations of traditional multi-channel fluorescence imaging in terms of crosstalk and unmixing, which cannot accurately image and quantify multi-color samples. This allows for precise quantitative analysis of single cells in the tissue microenvironment in situ with multiple targets. Additionally, it features multi-level tissue image recognition and tissue-like flow analysis capabilities, accurately identifying single cells and specific structural regions (such as glands, tumor areas, blood vessels, bronchi, etc.) in complex tissues. It performs localization, qualitative, and quantitative analysis at multiple levels, including single cells, tissue structures, and cellular spatial information. This includes quantitative analysis of staining intensity and morphology of fluorescently labeled proteins, nucleic acids, and other components; sample cell subtype analysis and screening based on fluorescence labeling and morphological parameters; and quantification of cell-to-cell positional relationships and spatial distribution.
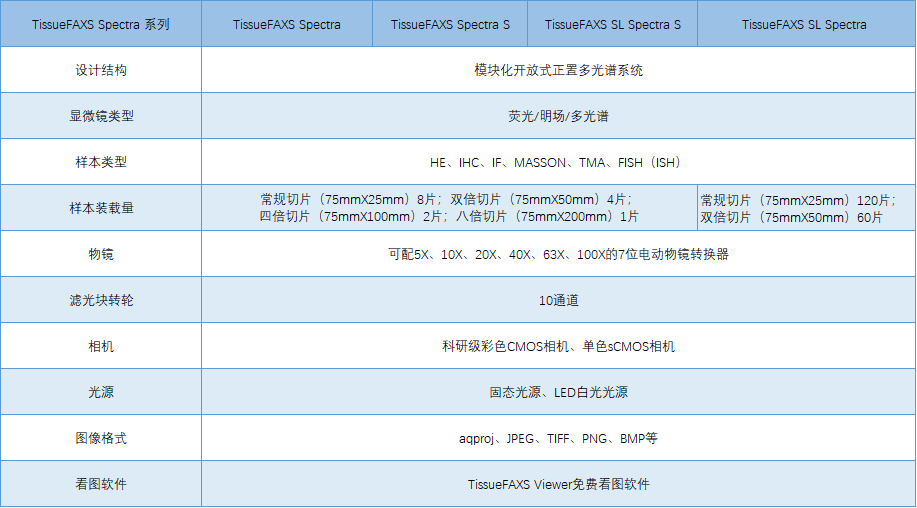
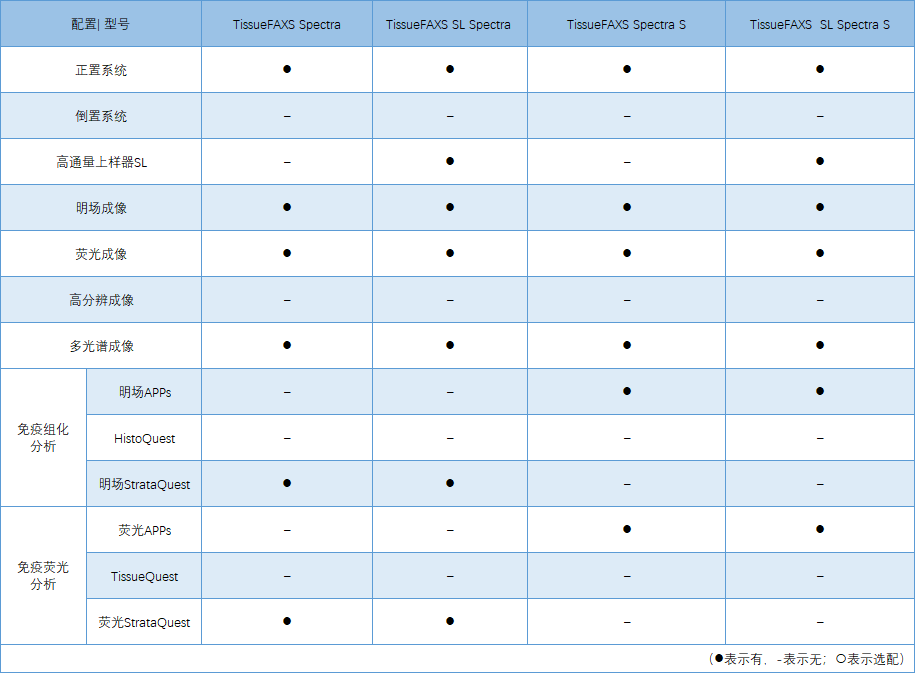
Independent 8-channel LED and laser hybrid light source
Fast spectral, full-spectrum continuous scanning and spectral splitting
Independent brightfield and spectral dual imaging module
Quickly view single-channel raw spectral calibration and splitting results
Customised independent spectral background deduction
Freely manage and call multiple spectral libraries