Immunotherapy provides alternative treatment for patients with advanced or metastatic cancer. Although immunotherapy prolongs overall survival and progression free survival, the response rate in head and neck squamous cell carcinoma (HNSCC) remains low. Infiltration into the tumor area, including the tumor bed and tumor nest, is a major step related to the function of cytotoxic CD8+T cells and is also one of the limiting factors of immunotherapy, especially for the efficacy of chimeric antigen receptor T cell immunotherapy (CAR-T) in solid tumors. However, little is known about the mechanisms that limit CD8+T cell infiltration.
In November 2023, the team led by Zhang Jianjun from the Ninth People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine published an article titled "Spatial and single cell transcriptomics reveal a cancer associated fibroblast subset in HNSCC that restricts inflammation and anti tumor activity of CD8+T cells" in Cancer Research. This study identified the trap of interferon induced MHC-IhiGal-9+CAF formation of CD8+T cells, providing insight into the complex network in the tumor microenvironment that regulates T cell infiltration and function.
In the article, spatial transcriptome analysis was conducted on HNSCC specimens with different immune infiltrates, and single-cell RNA sequencing was performed on five pairs of tumors and adjacent tissues, revealing specific tumor associated fibroblast (CAF) subpopulations associated with CD8+T cell infiltration limitation and dysfunction. These CAFs exhibit high expression of CXCLs (CXCL9, CXCL10, CXCL12) and enrichment of major histocompatibility complexes class I (MHC-I) as well as galectin-9 (Gal-9). The proportion of MHC-IhiGal-9+CAFs is negatively correlated with the abundance of TCF1+GZMK+subgroups in CD8+T cells. Gal-9 on CAFs induces dysfunction of CD8+T cells and reduces the proportion of tumor infiltrating TCF1+CD8+T cells. In summary, the identification of MHC-IhiGal-9+CAFs has advanced our understanding of the precise role of CAFs in cancer immune evasion and paved the way for more effective HNSCC immunotherapy.
Experimental part
In order to clarify the specific impact of CAFs on head and neck cancer, the authors of the article chose to use in situ spatial transcriptome technology to analyze the transcriptome expression related to the tumor microenvironment and tumor interior. The advantage of this technology is that it can indirectly reflect the expression of a large number of cell markers through spatial location limitation and transcriptional sequencing methods. However, due to limitations in detection accuracy and transcriptome level research, it is not yet possible to achieve the detection and analysis of true protein markers at single-cell resolution.
Therefore, based on spatial transcriptome technology, researchers need to use tissue slice multiplex immunofluorescence technology to validate in situ transcriptome level data through precise single-cell recognition algorithms, tissue recognition algorithms, and spatial distribution relationship algorithms.
The most noteworthy highlight of the author's application of multi immunofluorescence quantitative analysis technology at single-cell resolution is the use of Tissue Cytometry technology in the final data validation stage, which not only achieved the study of functional changes in TCF1+GZMK+CD8+T cells induced by Gal-9+CAFs in the tumor microenvironment, but also pioneered the use of the same frozen tissue sections as in situ spatial transcriptome analysis, even consecutive frozen sections adjacent to each other.
Compared to tissue sections fixed in formalin and embedded in paraffin, frozen sections can retain more tissue nucleic acid/protein components, but their tissue structure is more fragile and their binding ability to glass slides is poor. So in traditional TSA technology, microwave heating boiling bleaching is required for more than ten minutes between each round of staining, which makes it impossible to use frozen sections as staining samples.
The Tissue Cytometry technology uses TG paired with a multi immunofluorescence staining kit, which shortens the bleaching time to just over ten seconds. It is also equipped with professional frozen section repair elution reagents, which provide milder elution and solve the problem of multi immunofluorescence staining on frozen sections.
On this basis, the accurate identification and quantification of single cells, AI recognition of tissue structure, and the ability to analyze cell tissue cell distance relationships (non spatial vector coordinates) obtained through real in situ pixel level are all necessary conditions for precise quantitative verification of protein level data possessed by Tissue Cytometry technology.
As the provider of Tissue Cytometry technology, we are also pleased to have completed the staining, continuous full spectrum scanning, spectral splitting, and data analysis of the corresponding 7-color frozen sections in an extremely efficient manner during the author's data supplementation phase.

Figure 1 Heterogeneity of spatial distribution and gene expression profiles of three immune types of HNSCC cells
B:Multiple immunofluorescence images of PANK (green), CD8 α (yellow), and α - SMA (purple) with exclusion type immunophenotypes and their regional magnification.
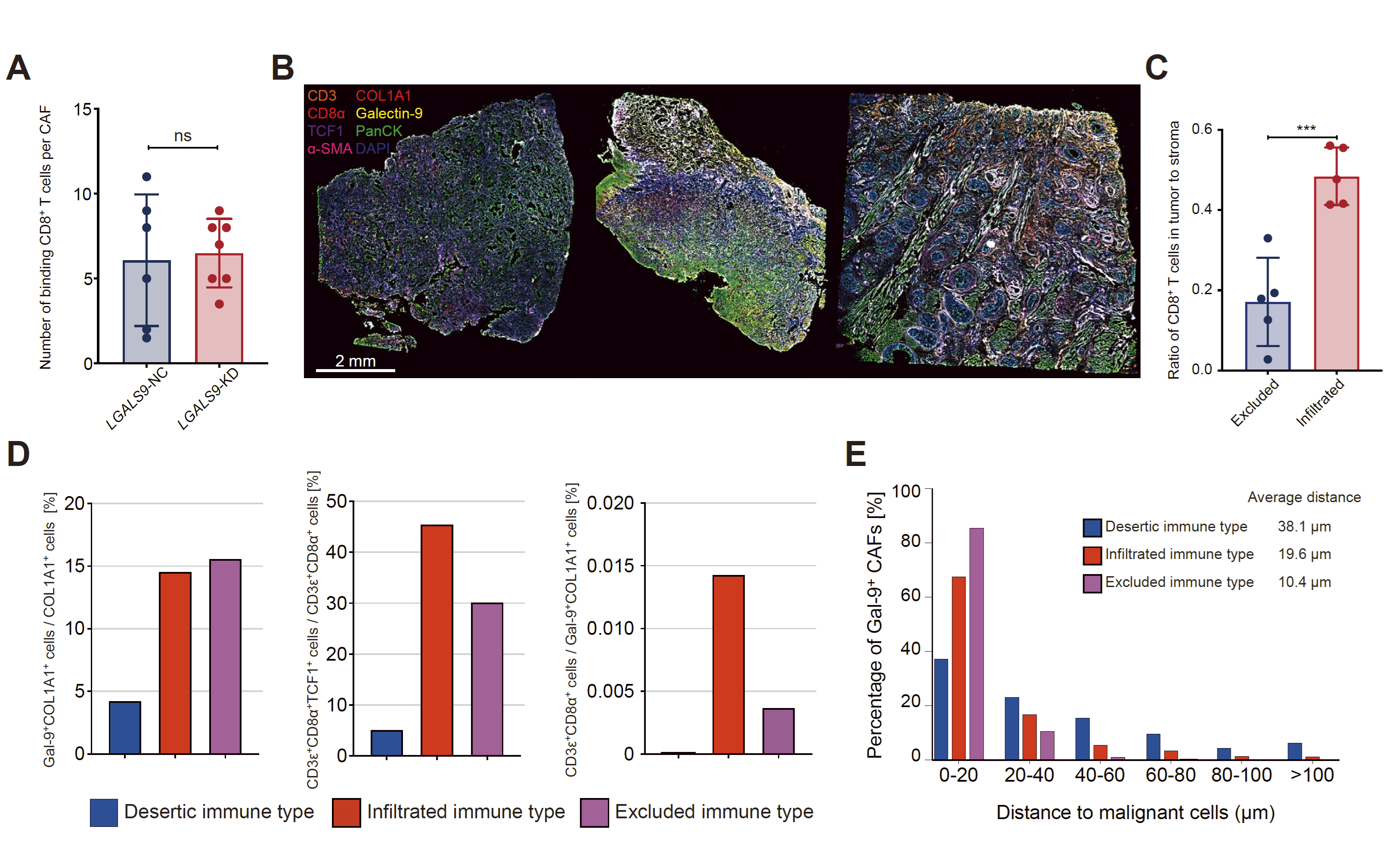
Figure 2 Galectin-9 is associated with impaired differentiation of TCF1+CD8+T cells.
B、Multiple immunofluorescence staining (CD3, CD8, α, PANCK, COL1A1, GAL-9, α - SMA) was performed on three immunophenotypic types of HNSCC, and spatial transcriptome sequencing was performed on identical specimens.
D,The proportion of TCF1+CD8+T cells in Gal-9+fibroblasts and CD8+T cells, as well as the ratio of CD8+T cells to Gal-9+fibroblasts, in three immune phenotype types of HNSCC. The proportion of Gal-9+CAF (Gal9+COL1A1+cells) within different distance ranges between these cells and malignant cells (Panck+cells).