Liver cancer is the second leading cause of cancer-related deaths among the most common malignant tumors worldwide, consisting of heterogeneous malignant tumor groups with different tissue characteristics and poor prognosis. Hepatocellular carcinoma (HCC) is the most common hepatocellular carcinoma. It is estimated that the incidence rate of primary liver cancer ranges from 600000 to 800000 every year, accounting for 5.6% of all human cancers, of which HCC accounts for 80% -90% of all primary liver cancer. The incidence rate of HCC is very high and tends to increase globally. The basic mechanisms of occurrence, development, and metastasis of primary liver cancer are still largely unknown.
Metabolic reprogramming of tumor cells and surrounding immune cells in the liver leads to an acidic tumor microenvironment. However, it is currently unclear how tumor cells adapt to this acidic stress during tumor progression.

On January 4, 2024, Gao Ping and Zhang Huafeng from the University of Science and Technology of China jointly published a research paper titled "Carnosine Regulation of Intracellular pH Homestasis Promotes Lysosome Dependent Tumor Immunolysis" online in Nature Immunology.
This study found that myopeptides, a mobile buffering metabolite that accumulates in tumor cells under hypoxia, regulate intracellular pH homeostasis and drive lysosome dependent tumor immune escape. A previously unrecognized isoform of creatine synthase, CARNS2, Promote peptide synthesis under hypoxic conditions.
Carnosine, as a mobile proton carrier, maintains the homeostasis of intracellular pH (pHi) by accelerating the migration and release of H+in the cytoplasm, thereby controlling the subcellular distribution, acidification, and activity of lysosomes. In addition, by maintaining lysosomal activity, myopeptides promote the degradation of nuclear transcription factor X-box binding 1 (NFX1), triggering galectin-9 and T cell-mediated immune escape and tumorigenesis. These findings indicate an unconventional mechanism of pHi regulation in cancer cells and demonstrate how lysosomes promote immune evasion, providing a basis for the development of liver cancer combination therapy strategies that utilize immune checkpoint blockade to disrupt pHi homeostasis.
Experimental part
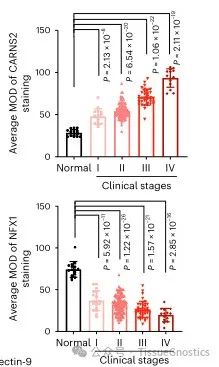
In order to further explore the pathological significance of CARNS2 in tumor immunity, the author of this article detected the expression levels of CARNS2, NFX1, and Galectin-9 in 10 pairs of human hepatocellular carcinoma tissues and adjacent non cancerous tissues. By using immunohistochemistry technology and Tissue Cytometry technology to quantitatively analyze the fluorescence intensity of CARNS2 protein and NFX1 protein, the results showed that CARNS2 protein was generally negatively expressed in normal liver tissue. However, as liver cancer progressed to higher clinical stages, the expression of CARNS2 protein gradually increased, while the expression of NFX1 showed the opposite trend.
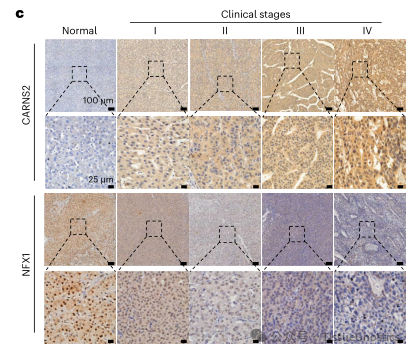
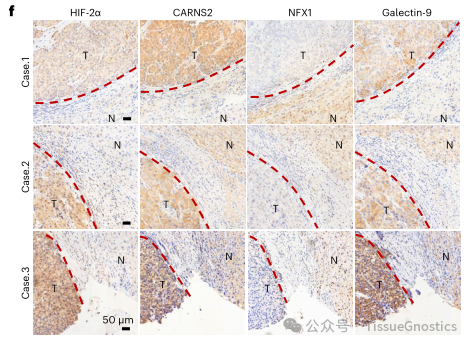
Subsequently, continuous slices were made of the same tissue, and immunohistochemical images were obtained using TissueFAXS. HistoQuest software was used to analyze the fluorescence intensity comparison of HIF-2 α, CARNS2, NFX1, and Galectin-9 proteins in the tumor and adjacent areas of hepatocellular carcinoma, and the correlation between the four proteins was confirmed.
The above section is the necessary foundation of this study. In order to objectively and clearly explain the premise of the experiment, the author of the article adopted classic scientific research ideas and intuitively performed tissue in situ section imaging and quantitative analysis through Tissue Cytometry technology. Combined with indirect methods such as flow cytometry and sequencing technology, comprehensive data results were obtained.
This study demonstrates that myopeptides can act as buffering agents to slow down transient pH turbulence and as a mobile proton carrier to accelerate the migration and release of H+within cells, in order to maintain pH homeostasis and lysosomal function. It was also found that the synthesis of myopeptides is catalyzed by a previously unknown isoform of myopeptide synthase, CARNS2, which promotes the expression of immune checkpoint protein galectin-9 by activating lysosomal degradation of transcription inhibitory factor NFX1. Experiments on in vivo allografts and in situ hepatocellular carcinoma (HCC) have shown that this CARNS2/NFX1/galectin-9 regulatory axis promotes immune evasion by inhibiting the abundance and function of CD8+T cells, and is highly correlated with liver cancer progression.
This study emphasizes that the reconnection of myopeptide metabolism in tumor cells is a key regulatory factor in peripheral T cell failure, as well as the potential therapeutic value of targeting pHi to promote anti-tumor immunity in tumors.