N-acetylaspartic acid (NAA) is one of the most abundant amino acid derivatives in the central nervous system (CNS) of mammals. It is broken down into L-aspartic acid and acetic acid by aspartate aminotransferase (ASPA) in oligodendrocytes. Acetic acid residues are believed to contribute to the synthesis of myelin. In addition, abnormal NAA metabolism is also associated with various neurological diseases, including white matter malnutrition and demyelinating diseases such as multiple sclerosis. Genetic defects in ASPA function can lead to Kanavan disease, characterized by elevated NAA levels, loss of myelin sheath and neurons, formation of large vacuoles in the CNS, and early death in childhood. Although the direct role of NAA in CNS is not yet clear, NAA derived acetic acid has been found to modify histones in peripheral adipose tissue, which is an epigenetic regulatory mechanism involved in cell differentiation.

On May 4, 2023, Professor Guangping Gao's team, director of the Hongrui Gene Therapy Center at the University of Massachusetts Medical School, published a research paper titled "Renewal of oligodendrocyte linear reversals dysmyelination and CNS neurogenesis through corrected N-acetylaspartate metabolism" in Progress in Neurobiology.
This article assumes that a lack of cell differentiation in the brain leads to demyelination and neurodegeneration in NAA metabolic disorders such as Canavan. Research has shown that the loss of ASPA function in mice disrupts myelin formation and shifts the transcriptional expression of neuronal and oligodendrocyte markers towards lower differentiation stages. After re expressing ASPA, these oligodendrocyte and neuronal lineage markers were improved or restored to normal, indicating that ASPA plays an important role in the decomposition of NAA during the maturation process of neurons and oligodendrocytes. In addition, the effect of ASPA re expression was inhibited in elderly mice, possibly due to limited recovery ability of neurons rather than oligodendrocytes. By restoring the expression of ASPA enzyme, NAA metabolism disorder can be improved, promoting the recovery of oligodendrocytes and neurons, thereby correcting pathological changes in the central nervous system.
Experimental part
This article uses the TissueFAXSiQ inverted high-resolution panoramic rapid scanning system from TissueGenomics to collect immunofluorescence images of panoramic brain tissue slices.
The experiment fluorescently labeled more than 10 types of RNA fragments, and further obtained quantitative analysis results at the single-cell transcriptome level at the confocal level, as well as corresponding morphological data of the cells, using TissueGenomics' TissueCytometry technology. Considering that the relevant data analysis is based on panoramic imaging and Z-axis panoramic deep imaging to ensure the objectivity and authenticity of the data, a new breakthrough has been achieved at the technical level.
In addition, Tissue Cytometry technology can also support imaging of tissue in situ with immunofluorescence staining of 30+or more, and can also meet the requirements of Z-axis panoramic deep imaging on the basis of panoramic confocal level clarity; On the dimension of single-cell quantification, deep analysis of molecular probes protein labeling tissue morphology spatial microenvironment distribution can also be completed. Whether it is in situ validation through transcription sequencing and other technologies, or as a standardized tool with a complete set of solutions, it can achieve more and more in-depth assistance goals for important cutting-edge research topics.
The effect of ASPA enzyme activity recovery on Nestin expression in affected brain regions
After ASPA overexpression, the number of Nestin positive cells in the mouse brain increased, especially in the most severely damaged brain regions such as thalamus, cerebellum, brainstem, and corpus callosum. This may indicate that ASPA overexpression promotes the activation and proliferation of neural stem cells or other types of stem cells, providing a cellular source for the repair of the nervous system.

Fig 1 After ASPA overexpression, Nestin mRNA+cells increased overall in the central nervous system and showed a region specific distribution.
(B)Nestin mRNA+cell counts in the cortex (CTX), striatum (STR), corpus callosum (CC), hippocampus (HC), thalamus (TH), midbrain (MB), cerebellum (CB), and brainstem (BS) regions.
(C)Nestin+mRNA cell counts in brain regions such as TH, CB, BS, and CC.
(D) Immunofluorescence images of Nestin+mRNA fluorescence positive cells in various brain regions 4 weeks after injection (circled in green).
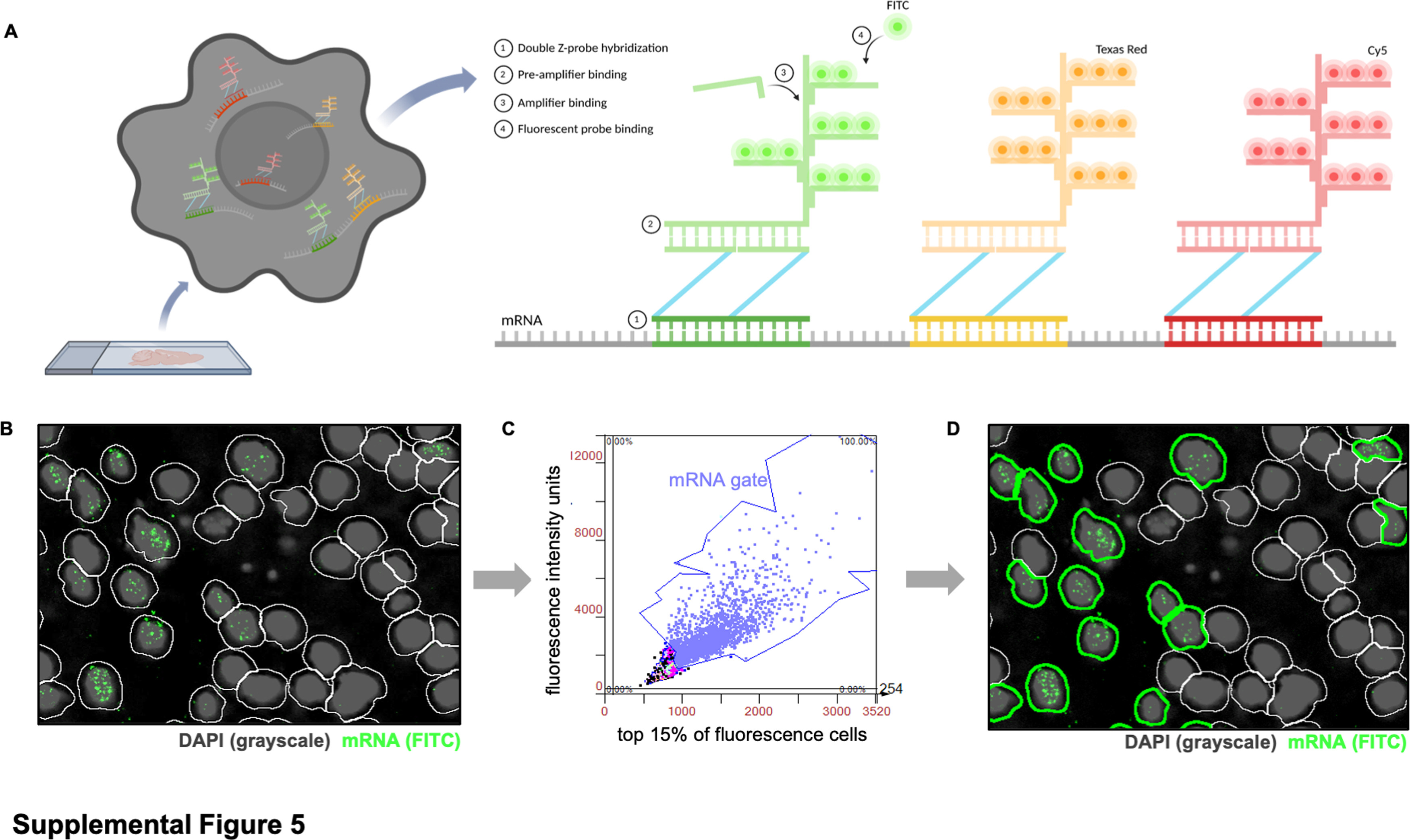
Spatial quantification analysis of single-cell RNA
Import tissue sample images of whole brain sagittal slices (3 per experimental group and time point) into StrataQuest software. Overlay subsequent multiple rounds of images through DAPI+cell localization, and use ROI circles to select regions of interest: cortex, corpus callosum, hippocampus, striatum, thalamus, midbrain, cerebellum, and brainstem. To analyze single cells that are positive for fluorescent mRNA targets, software was used to identify the nuclei of DAPI stained tissues. Next, generate a contour (white) around each nucleus to mark the boundaries of each cell body (Figure S5B). Then, in order to avoid false positive counts caused by tissue spontaneous fluorescence, detection parameters of maximum fluorescence intensity (x-axis) and total fluorescence intensity units (y-axis) were set for each channel/mRNA target, and the same settings for the same channel were applied to all experimental groups. Then, threshold division of cells was performed based on the highest fluorescence intensity of mRNA dots (Figure S5C). Finally, export the counts of all cells (white and green outlines), total mRNA+cells (only green outline) (Figure S5D), and total DAPI+cells.