Diffuse gliomas, including isocitrate dehydrogenase (IDH) - wild-type (wt) glioblastoma (GBM), IDH mutant astrocytoma, and oligodendroglioma, are the most common malignant brain tumors in adults. Tumor associated macrophages (TAMs) are the dominant immune infiltrating cells in diffuse gliomas. TAMs directly interact with malignant cells, promote tumor progression, and serve as architects of the immunosuppressive microenvironment. Therefore, TAMs have become an attractive therapeutic target.
As a hallmark of solid tumors, hypoxia is associated with tumor necrosis, neovascularization, and the production of various inflammatory and chemokines. The clues of hypoxic tumors are limited in space and may guide genomic instability, transcriptional adaptation, and metabolic reprogramming of tumors and niche cells. In addition, enhanced TAM recruitment and immunosuppressive tumor myeloid cell interactions in the hypoxic niche enhance the resistance of gliomas to chemotherapy and immunotherapy. This prompted researchers to determine how hypoxic niche cues distort hypoxic TAM features and how hypoxic TAMs interact with other cells to promote tumor progression.
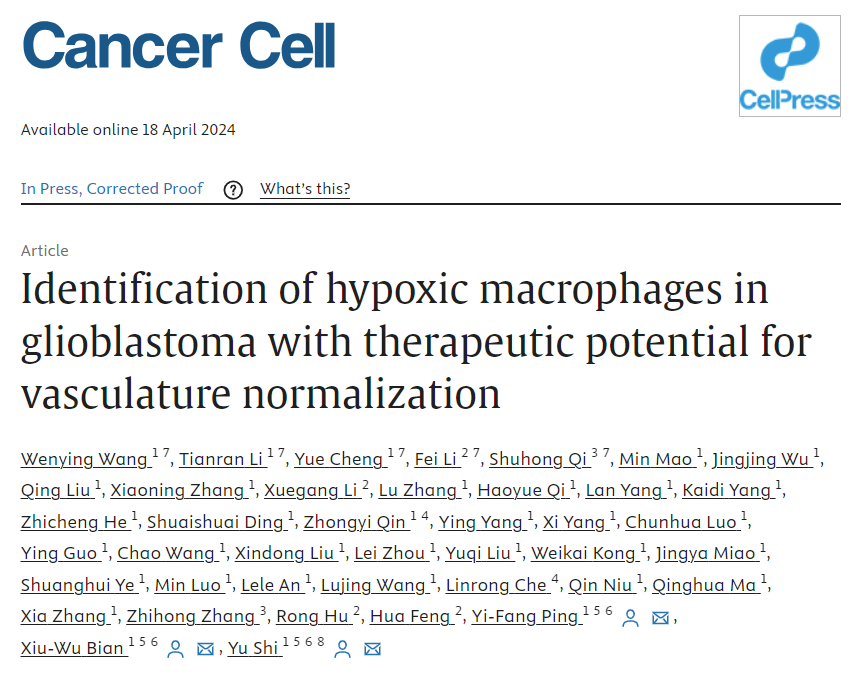
On April 18, 2024, a team led by Academician Bian Xiuwu, Associate Professor Shi Yu, and Professor Ping Yifang from the Clinical Pathology Research Institute of the First Affiliated Hospital of the Army Medical University (Chongqing Southwest Hospital), together with Associate Professor Li Fei from the Brain Glioma Medical Research Center and Associate Professor Zhang Zhihong from the Neurosurgery Department of the First Affiliated Hospital of the Army Medical University, and Associate Researcher Qi Shuhong from the Wuhan Optoelectronics National Research Center of Huazhong University of Science and Technology, published an article titled "Identification of hypoxic macrophages in glioblastoma with therapeutic potential for vascular normalization" in Cancer Cell (IF=50.3). Revealed the presence of hypoxic macrophages in glioblastoma and their potential role in tumor vascular normalization therapy.
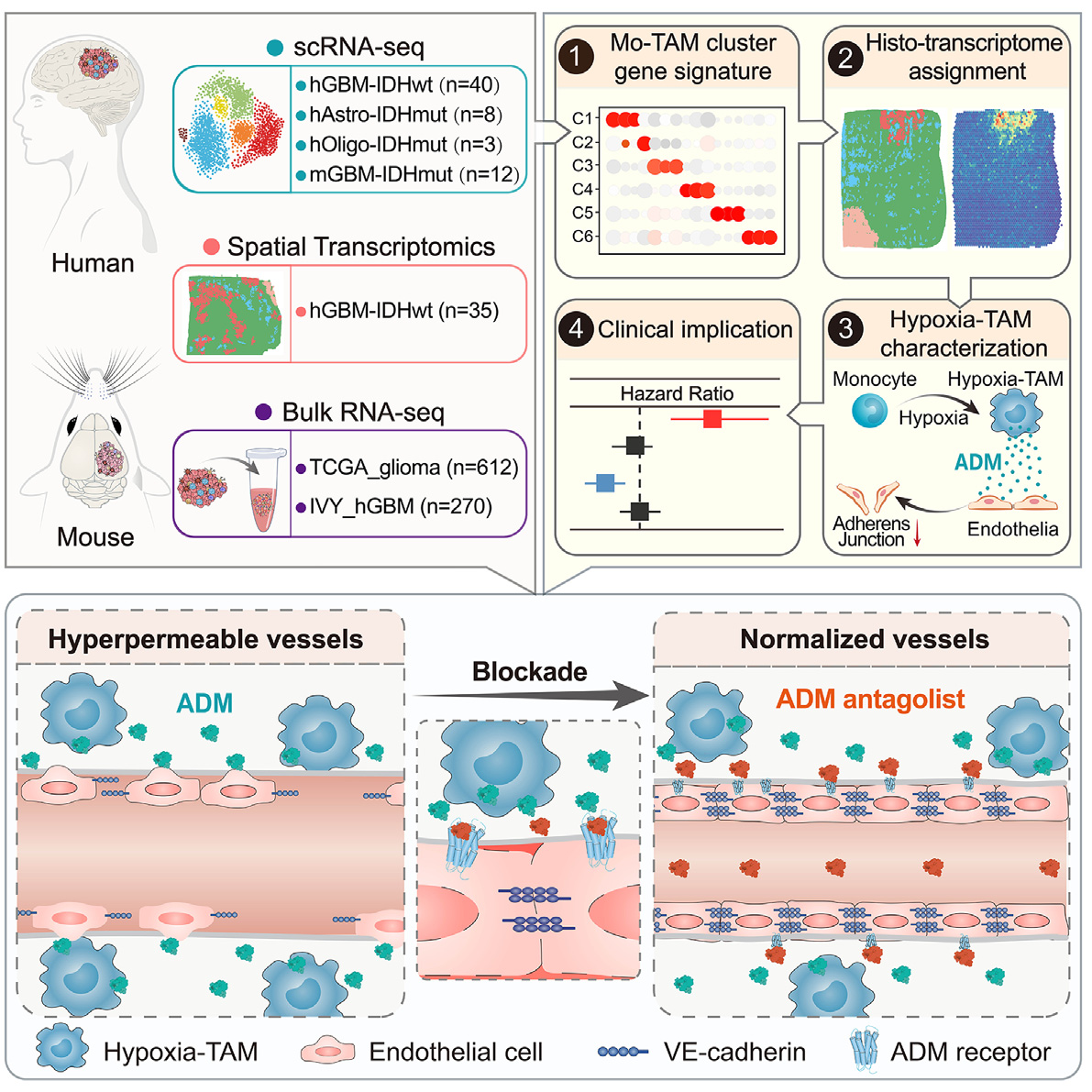
In diffuse glioblastoma, tumor associated macrophages (Mo TAMs) derived from monocytes infiltrate densely with significant heterogeneity. Through single-cell transcriptomics, the authors plotted the spatial resolution transcriptional landscape of Mo TAMs in 51 patients with wild-type glioblastoma or IDH mutant glioblastoma carrying isocitrate dehydrogenase (IDH). A Mo TAM subgroup located in the necrotic peripheral area was characterized using in situ efficacy validation techniques in vivo, and the hypoxic response characteristics were obtained under the influence of hypoxic niche cues. Hypoxia TAM activates the paracrine signaling of adrenal medulla and disrupts endothelial cell adhesion connections, thereby stimulating a highly permeable neovascularization and hindering drug delivery in glioblastoma xenografts.
Therefore, by genetic elimination or drug blockade of adrenomedullin produced by hypoxia TAM, vascular integrity can be restored, the intratumoral concentration of anti-tumor drug dabrafenib can be increased, and the combination therapy effect can be achieved. An increase in the proportion of hypoxia TAM or expression of adrenomedullin indicates high tumor vascular permeability and poor prognosis of glioblastoma. The article highlights the diversity of Mo TAM and spatial niche guided Mo TAM reprogramming, suggesting potential therapeutic approaches for normalizing tumor blood vessels through hypoxia TAM.
In situ transcriptome technology not only enables in-depth identification of differentially expressed genes through sequencing techniques, but also provides spatial localization data of tissue in situ, exploring the functional relationships of key genes from distance features. However, at the same time, the accuracy of existing in situ transcriptome sequencing techniques has not yet reached subcellular resolution, and there is still a lack of reliable data consistency comparison schemes at the protein level carried by cellular functions.
The author of this article extensively applied Tissue Cytometry technology, using antibodies to fluorescently label multiple target proteins in the sample, identifying vascular morphology, tumors and their necrotic areas, and constructing and exploring the relationship between cells and blood vessels in the hypoxic microenvironment.
This approach of in situ data validation not only fills the gap in in in situ transcriptome technology, but also further enhances the reliability of the data, providing strong support for the viewpoint of the article.
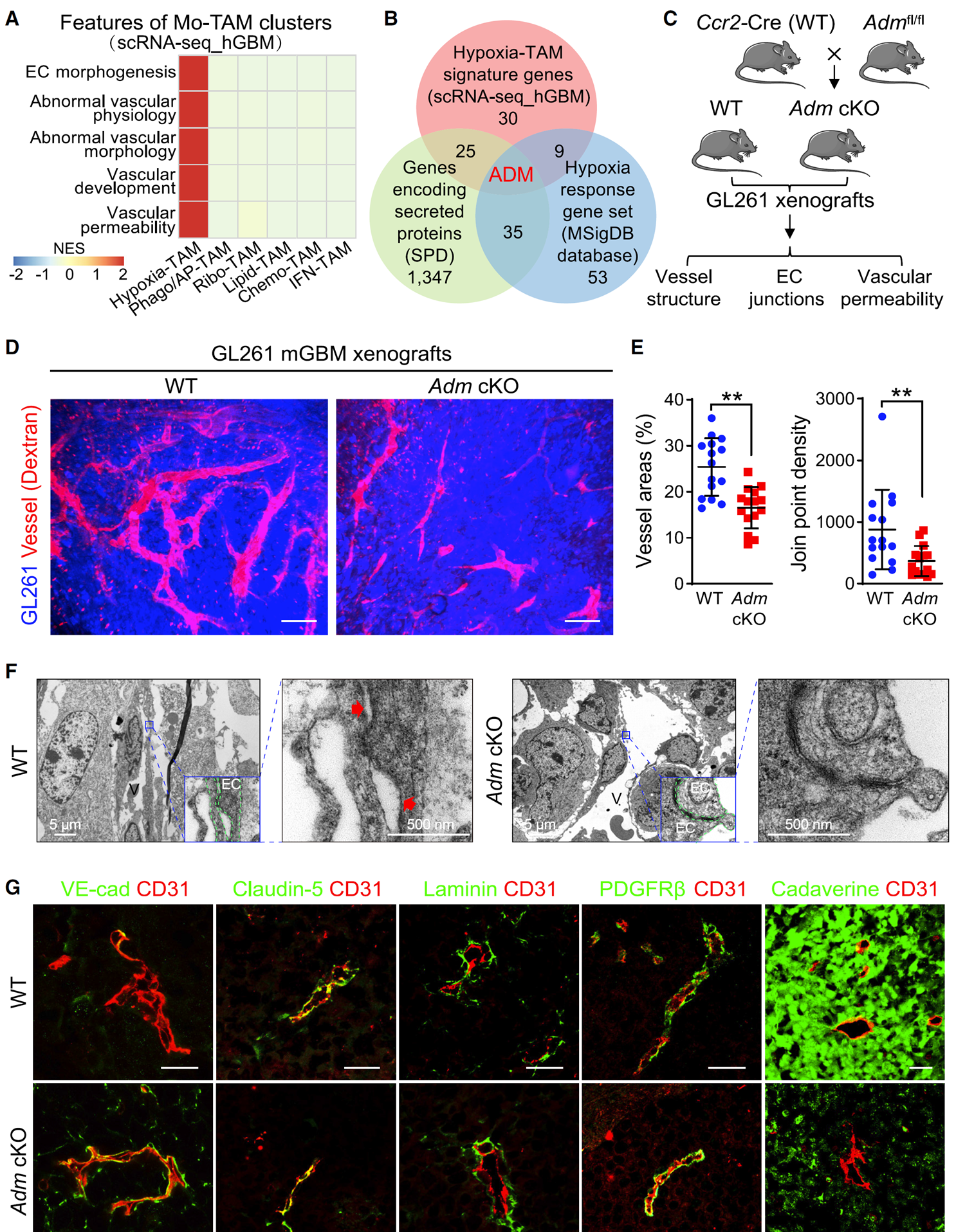
Figure 2 ADM knockout can restore vascular integrity of mGBM xenografts
(D and E) In vivo imaging of tumor vasculature labeled with dextran (D) and quantification of vascular structure in WT and Adm cKO xenografts (E) (n=15 images/group). Scale bar, 100 micrometers.
(G) VE cadherin in xenografts claudin-5、 Laminin PDGFRb、 Immunostaining of corpse alkaloids and CD31 (n=15 images/group). Scale bar, 25 microns.
Using the TissueFAXS Spectra panoramic multispectral tissue scanning quantitative analysis system, the authors employed multiplex immunofluorescence technology to perform VE cadherin analysis on Adm cKO xenograft tissue samples, Perform multiple immunostaining of claudin-5, laminin, PDGFRb, CADAverine, and CD31, and obtain multi-color fluorescence images. Using StrataQuest software, non-specific background is first removed from multi-color fluorescence images, and vascular and other marker staining is optimized. Then, AI classifier is used for specific recognition of blood vessels, and protein expression of various markers is quantitatively analyzed.
The results showed that in ADM cKO xenografts, the expression of endothelial adhesion junction marker vascular endothelial (VE) - cadherin was basically restored, while the expression of tight junction marker claudin-5, pericyte marker platelet-derived growth factor receptor, and vascular basement membrane marker laminin was not affected by ADM knockout. The evaluation of tumor vascular permeability using CADAVINE showed that compared with wild-type xenografts, ADM cKO xenografts had significantly reduced CADAVINE exudation area. These data indicate that knocking out ADM selectively restores the integrity of tumor blood vessels and reduces vascular leakage in mGBM xenografts.
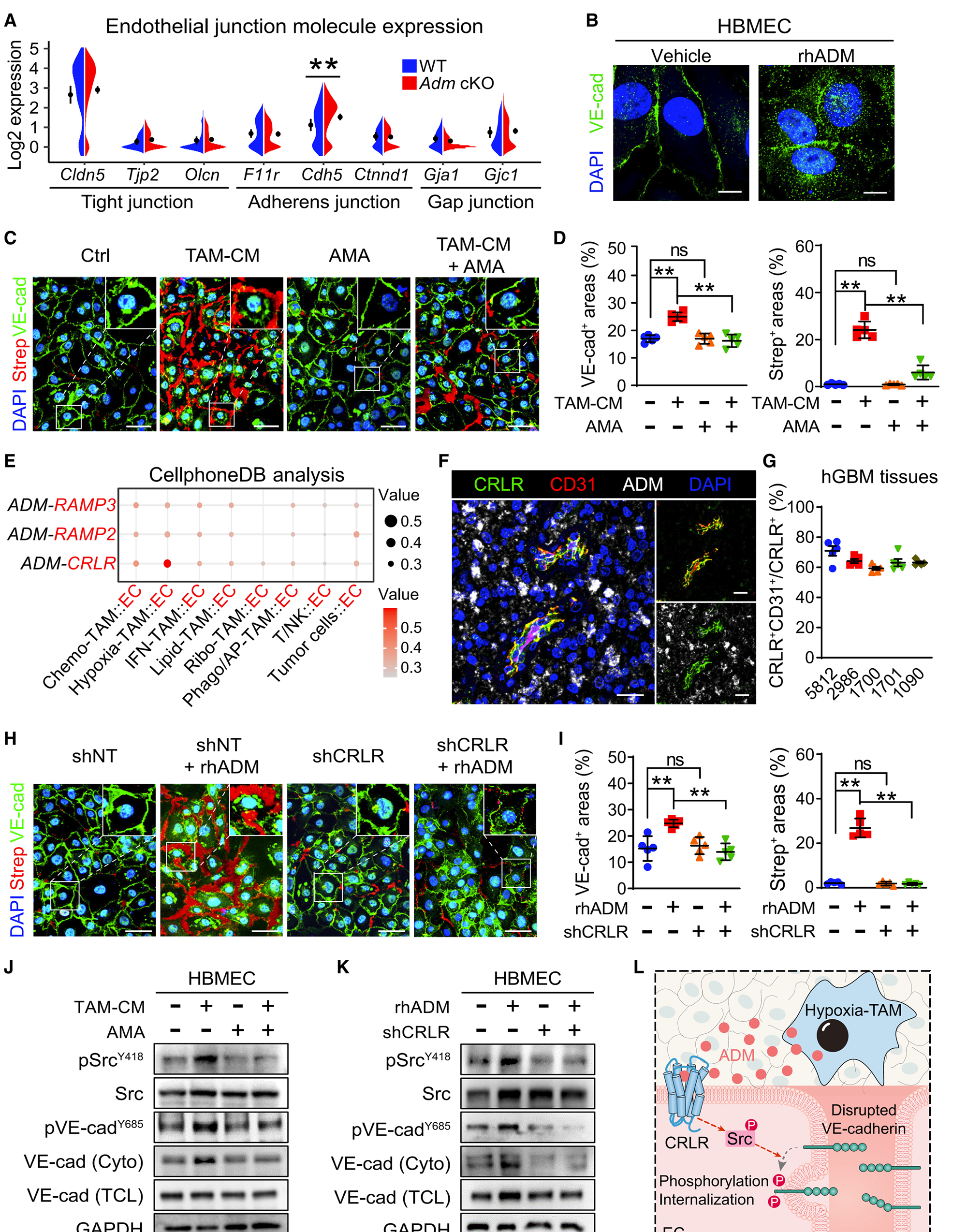
Figure 3 ADM disrupts endothelial cell adhesion junctions by activating the CRLR signaling pathway
(B) Immunostaining of VE cadherin in HBMEC treated with rhADM or carrier. Scale bar, 10 micrometers.
(C 和 D)Immunostaining of VE cadherin and Strep (C) in HBMECs treated with hypoxia TAM-CM and/or AMA, as well as quantification of VE cadherin and streptococcal regions (D) (n=5 samples/group). Scale bar, 20 microns.
The author used multiple immunofluorescence techniques to perform multiple immunostaining of ADM, CRLR, and CD31 in hGBM tissue and obtained images. Tissue Cytometry was used to quantitatively analyze the co localization expression of immunostaining. The results confirmed that the co localization of CRLR and endothelial cell marker CD31 was accompanied by upregulation of ADM signal around the necrotic area of hGBM. ADM disrupts the adhesion connections of endothelial cells by activating CRLR signaling.
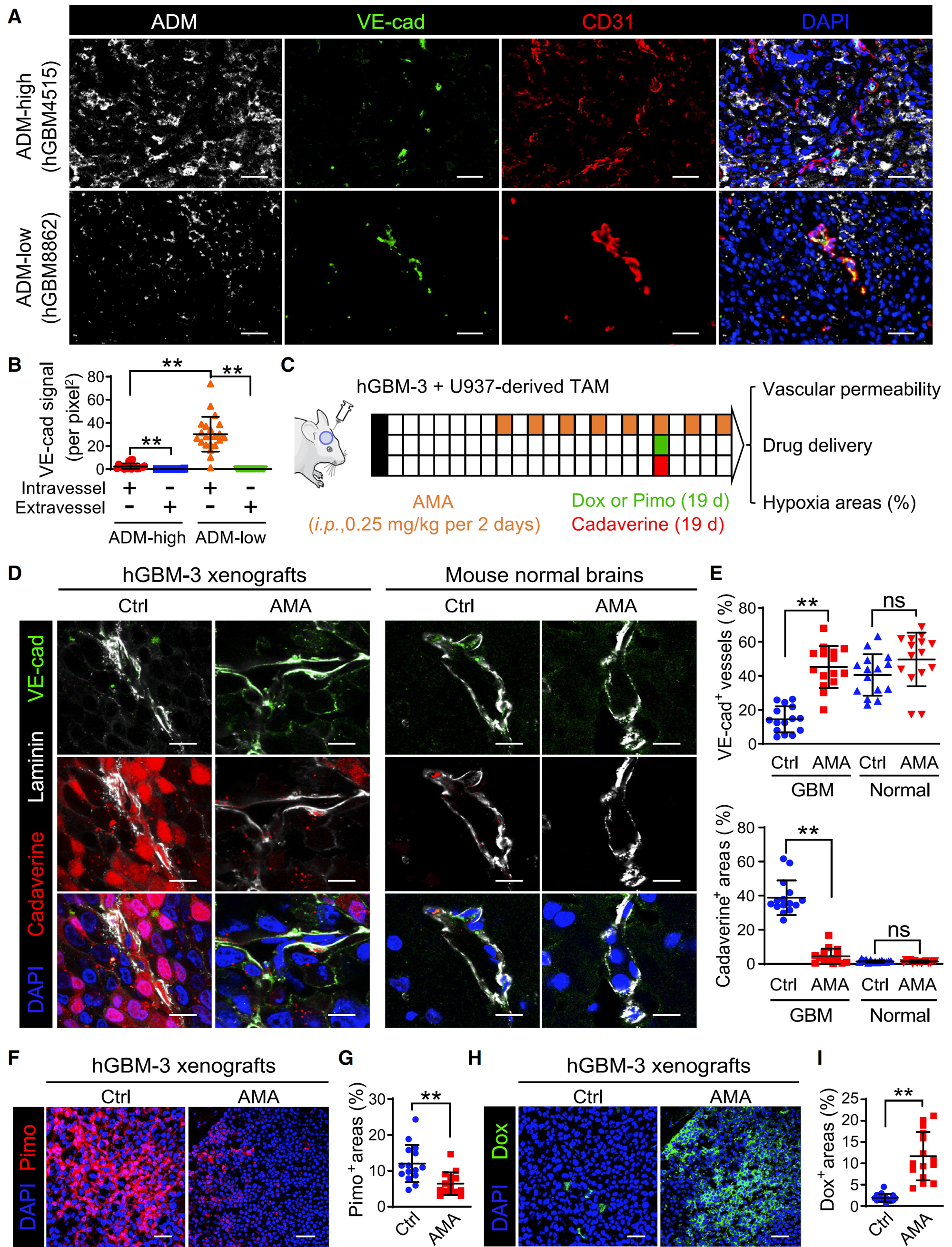
Figure 4 AMA treatment can normalize the tumor vascular system in hGBM xenografts
(A)Immunostaining of ADM, VE cadherin, and CD31 in hGBM tissue. HGBM (n=41 cases) was stratified based on the proportion of ADM cells. Scale bar, 50 micrometers.
(B) Quantification of VE cadherin signaling in hGBM tissue (n=41 cases, 5 images/case).
(D 和 E)Immunostaining of VE cadherin, cadavers, and laminin (D), as well as the percentage of exudate areas and VE cadherin blood vessels (E) in hGBM-3 xenografts and normal mouse brains (n=15 images/group). Scale bar, 10 micrometers.
(F 和 G)Immunostaining of Pimo (F) and percentage of Pimo tumor area (G) in xenografts (n=15 images/group). Scale bar, 50 micrometers.
(H 和 I)Immunostaining of the percentage of Dox (H) and Dox tumor area (I) in xenografts (n=15 images/group). Scale bar, 50 micrometers.
The author used multiple immunofluorescence techniques to perform ADM on hGBM tissue, VE cadherin and CD31 immunostaining were used to quantitatively analyze VE cadherin signaling in hGBM tissue using StrataQuest software, and hGBM tissue was stratified based on the proportion of ADM+cells.
Through VE cadherin, Multiple immunostaining of cadherin and laminin was performed using StrataQuest software to quantify the area of cadherin effusion and the percentage of VE cadherin+blood vessels in hGBM-3 xenografts and normal mouse brains, respectively. AMA has been validated to restore VE cadherin expression, preserve endothelial connections, and reduce cadherin effusion in tumor transplants, but its impact on the normal cerebral vascular system of mice can be ignored. Quantify the percentage of Pimo+and Dox+tumor areas in xenografts using immunostaining with Pimo and Dox and StrataQuest software. The results showed that AMA treatment reduced tumor hypoxia and enhanced drug delivery. In xenografts receiving AMA, pyrazole labeled hypoxic areas were significantly reduced, while doxorubicin perfusion areas were increased.
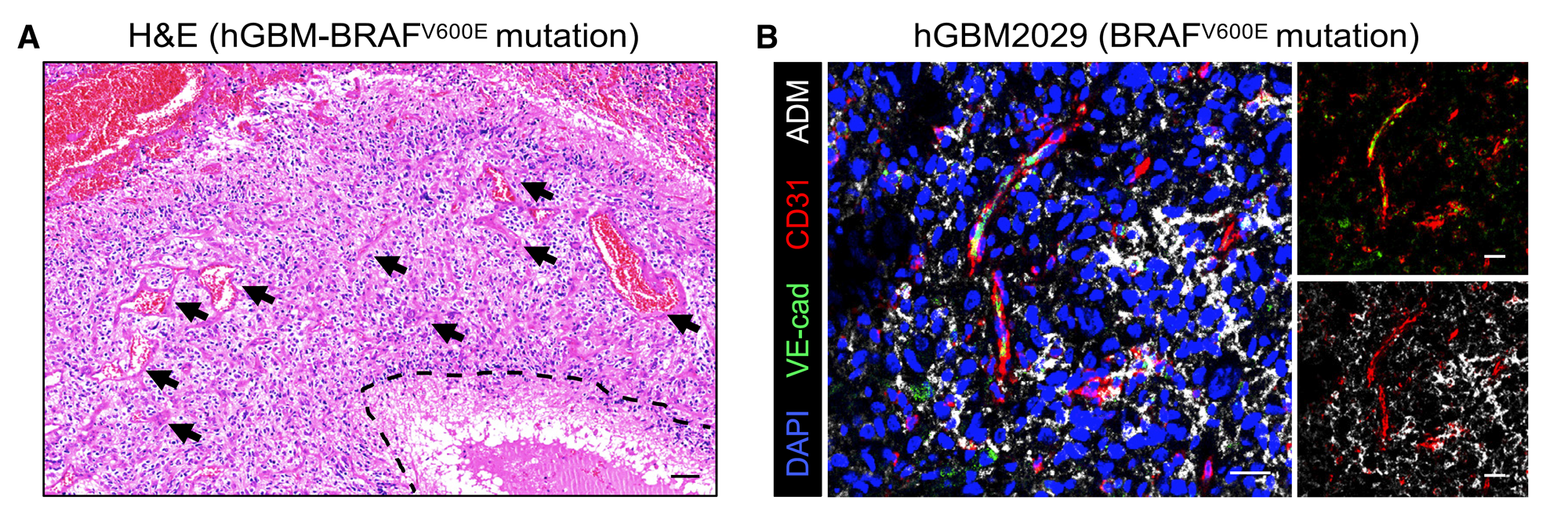
Figure 5 AMA treatment has improved the administration and therapeutic efficacy of dabrafenib
(A 和 B)H&E staining (A) and immunostaining of VE cadherin, CD31, and ADM (B) in BRAF mutant hGBM. The blood vessels in the necrotic area and the surrounding necrotic area are highlighted with dashed lines and arrows, respectively. Scale bar, 100 microns (A) or 25 microns(B)。
Through multiple immunostaining and fluorescence imaging of VE cadherin, CD31, and ADM in the hGBMs tissue of BRAFV600e mutant, it was observed that the tissue exhibited significant necrosis, lacked VE cadherin, and had abundant blood vessels and a large number of TAMs producing ADM.